In this article, TherapeutAix has come together with one of our partners, OracleBio, a provider of Quantitative Digital Pathology services supporting pharma and biotech R&D, to provide our collective thoughts on the use and value of the pre-clinical bleomycin model in drug development for Idiopathic Pulmonary Fibrosis (IPF).
IPF is a progressive lung disease with unknown cause, characterised by deposition of extracellular matrix (‘fibrosis’). The two approved drugs for this condition, nintedanib and pirfenidone, slow, but neither reverse nor halt, disease progression and tissue remodelling. The specific disease cause is still unknown (hence the ‘idiopathic’). Development of drugs that attenuate this disease has proven difficult, as it is unclear which of many mechanism(s) to target. That’s why many projects make use of the multi-mechanism ‘bleomycin model’. In this model, bleomycin is instilled into the lungs of mice or rats, leading to deposition of extracellular matrix and fibrotic remodelling of the lungs. This outcome is similar to findings in lungs of IPF patients, and it could be argued that if a drug reduces fibrotic remodelling in this model, it can be expected that it shows similar effects in patients. in this model, it can be expected that it shows similar effects in patients.
Incomplete understanding of IPF in preclinical models
TherapeutAix has worked with many clients and providers on this model and can help with setting up the study in an optimal way. The design of the bleomycin study needs to reflect the target mechanism, and the right dosing regimen is crucial. Different providers have their preferred model; and different pharma and biotech companies favour data from one over the other. There are also a number of challenges with the bleomycin model itself. First, and possibly most importantly, lung fibrosis in this model has a clear cause (the bleomycin) and is not idiopathic, and of course fibrosis in patients could arise via different mechanisms. Second, while IPF is a chronic disease that develops over years, the bleomycin model induces lung changes quite quickly: around 10 days with predominantly inflammation, and then another 10 days with fibrosis (duration and overlap of these phases depend on the protocol used). And third, unlike human disease, the rodent bleomycin model will eventually resolve. Finally, it is quite difficult, if possible at all, to quantitatively benchmark drug candidates in this model against the approved drugs, which, to make things more difficult, sometimes do not work in this model.
While some of the readouts in the bleomycin model can be mode-of-action specific, almost always it is of key interest to assess a compound’s effect on fibrosis. This can be crudely assessed by lung weight or more directly evaluated by measuring activation of profibrotic pathways e.g. in fibroblast cells, and, directly, by assessing deposition of extracellular matrix proteins like fibronectin or collagen. These structural proteins are usually assessed using biochemical assays in lung tissue homogenates. A key downside of this approach is that any spatial information is lost because fibrotic remodelling and any effect on it are ‘averaged’ over the tissue specimen. Although somewhat rarely used, could modern image analysis methods provide a superior evaluation method?
In pre-clinical lung fibrosis models, a key aim is the quantification of the fibrotic and non-fibrotic components across different whole lobe sections to gauge disease development or therapeutic response. Histological stains like Picrosirius Red (PSR) or Masson’s Trichrome can be used to highlight the different structural components of the lung tissue, including collagen deposition and cell nuclei within fibrotic regions, and can help with the segmentation of lesion areas from healthy tissue. The example below (Figure 1) shows a Whole Slide Image (WSI) containing PSR stained Pulmonary tissue sections from two different lobes containing varying amounts of Fibrosis.
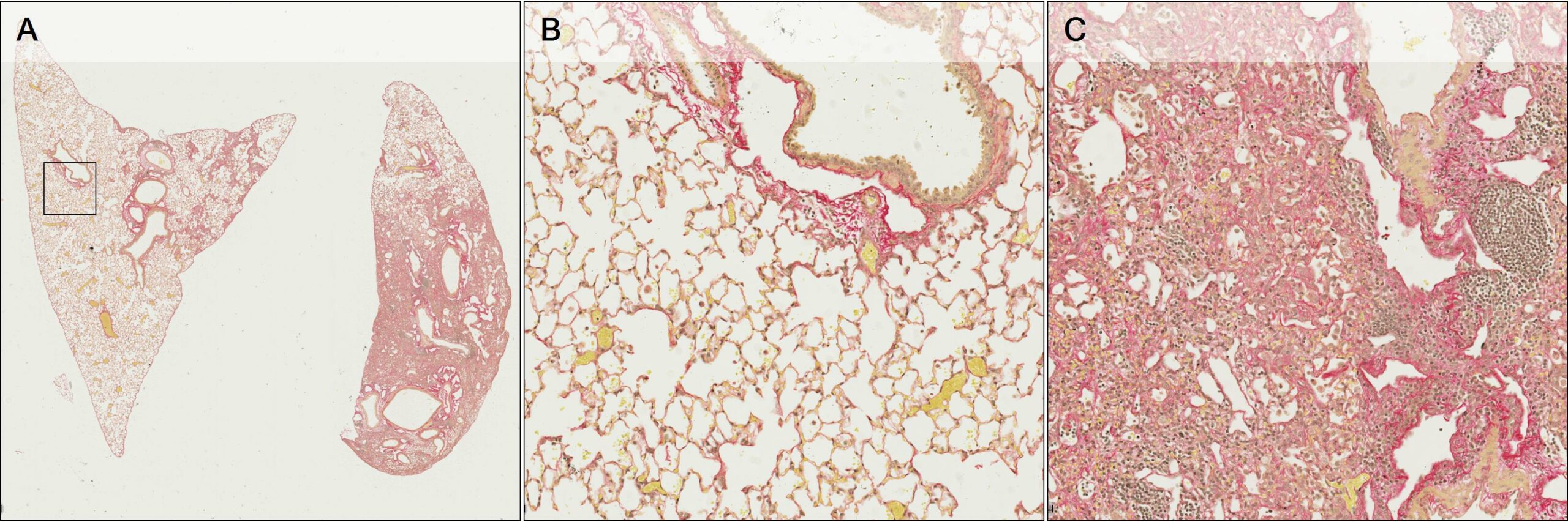
Figure 1 – A: WSI containing Pulmonary tissue sections stained with PSR. B and C: Magnified areas showing Normal Parenchyma and Fibrotic lesion.
Using Machine Learning to complement analyses
When planning a pre-clinical fibrosis study, whether the focus is lesion content, collagen quantification, cell density or in quantifying a specific cell type such as the CD68 positive population to investigate the immune alterations, quantitative pathology techniques can help improve accuracy and turnaround time. Each study can present with its own staining or morphological challenges, OracleBio therefore implement Machine Learning capabilities with Deep Learning (DL) algorithms, which address the vast heterogeneity in Fibrosis model tissue and allows us to generate robust data. Furthermore, OracleBio have expert pathologists who can complement image analysis results with qualitative Ashcroft scoring.
Image analysis workflow
From an image analysis perspective, it is important to understand the different components present in the tissue. In the magnified area above (Figure 1B), normal lung parenchyma (alveolar space) is visible alongside bronchial structures and blood vessels of various size. In Figure 1C, there is evidence of fibrotic lesion with inflammatory cell infiltration and dense collagen deposition, with most normal lung parenchyma obliterated.
When working with a processed tissue and a digitized image, the section will most likely present artefacts, therefore, OracleBio’s image analysis workflow starts with a thorough QC of each image to assess its suitability for analysis. This involves checking scan quality (focus issues), tissue quality (section folds, damage, artefacts etc) and stain quality (intensity and consistency across the sample).
The second step involves the segmentation of each tissue section into defined regions of interest (ROI) including fibrotic lesion, non-fibrotic lung parenchyma, basal collagen structures (bronchioles and prominent vascular structures) and glass. This allows for direct quantification of lesion amount and content per section, excluding any non-fibrotic basal collagen structures in the tissue. To do this, OracleBio use image analysis software, such as Indica Labs Halo®, which contains a Halo AI DenseNet classifier, a neural network suited for detailed tissue segmentation. They manually annotate training regions per ROI, making sure to incorporate as much context as possible, and use them as input data for the algorithm, which is trained until an accurate identification of each tissue component is achieved (see examples in Figure 2).
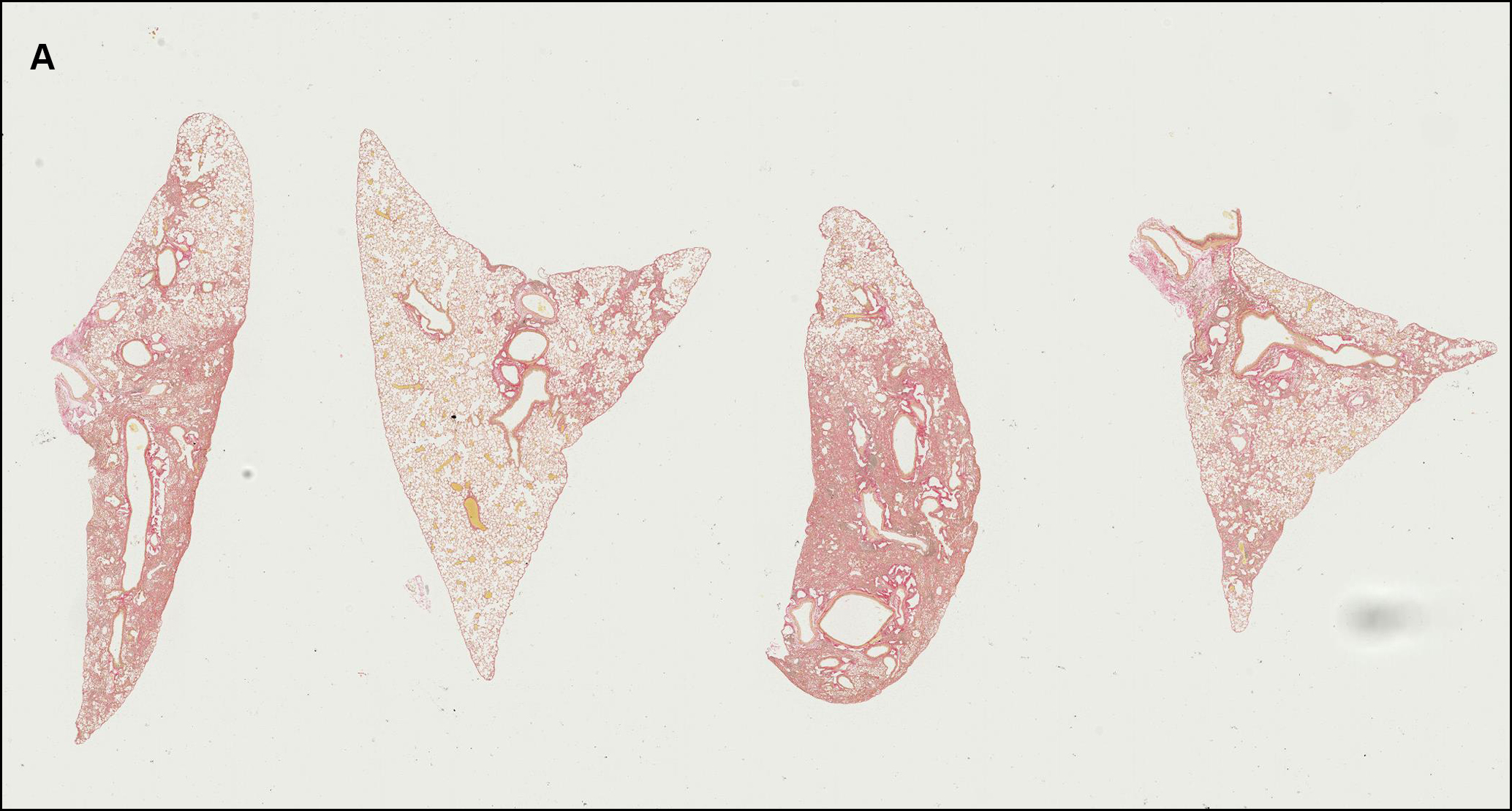
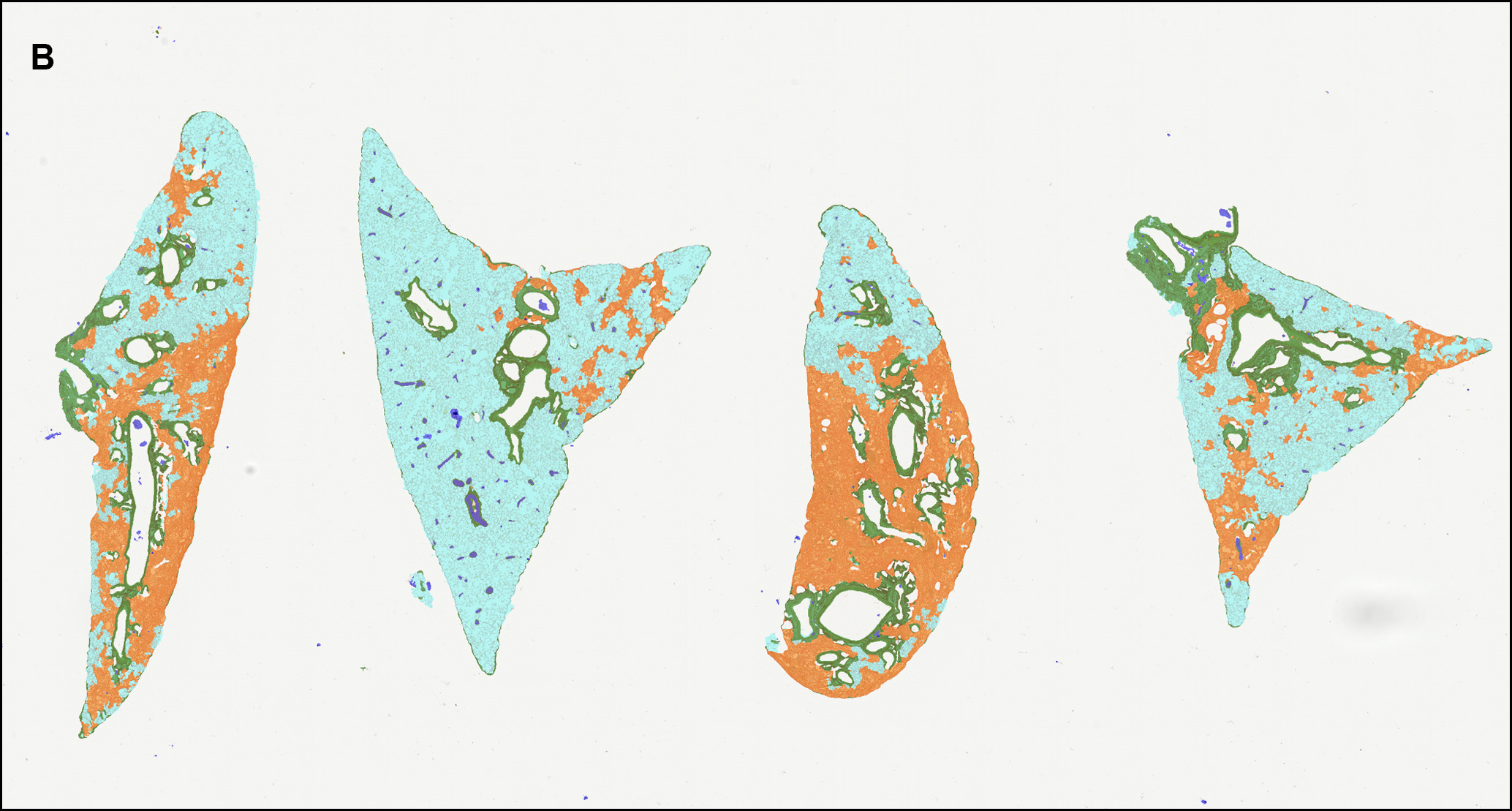
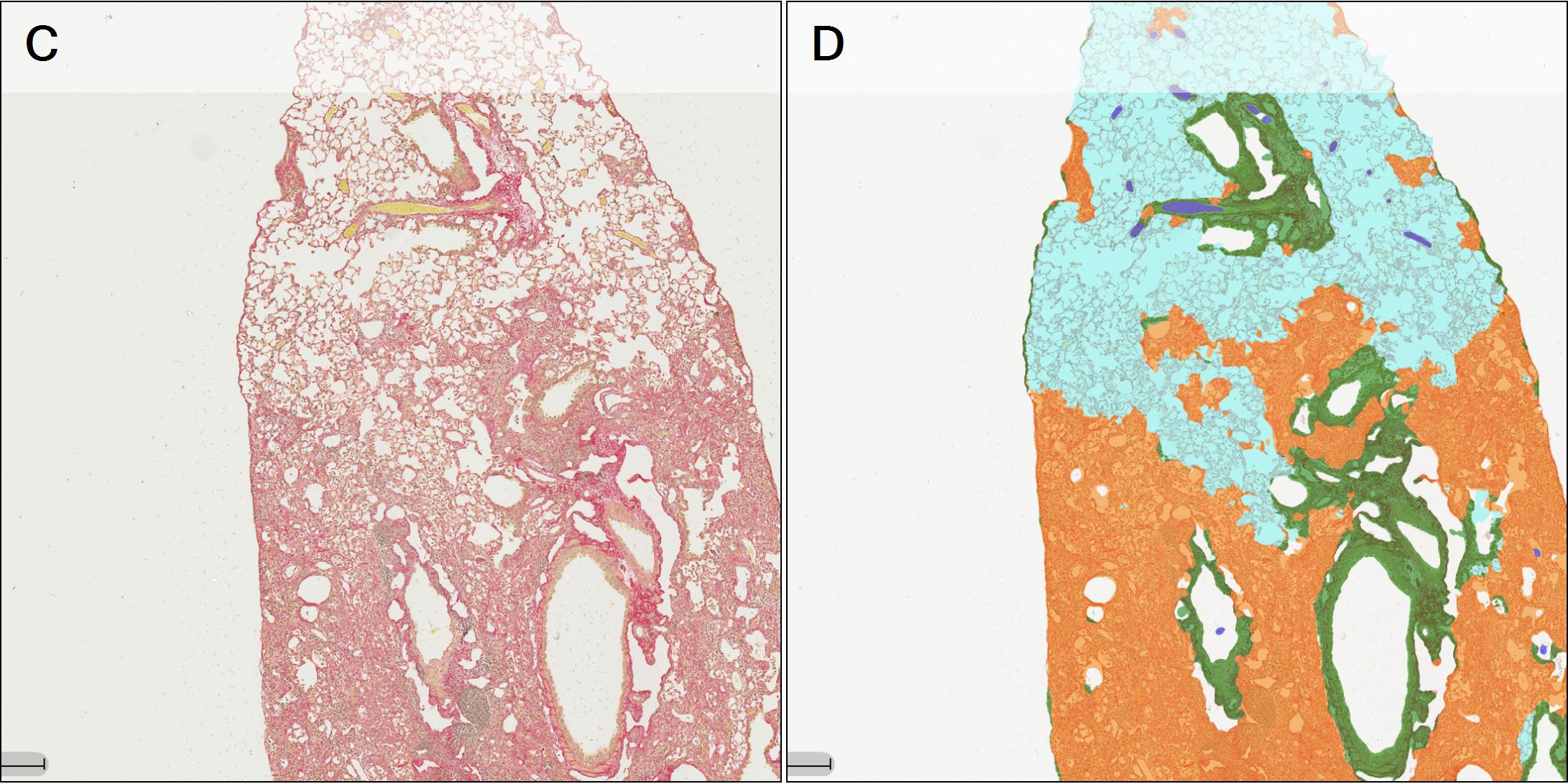
Figure 2 – A: WSI showing 4 PSR stained tissue sections (lobes). B: ROI classifier overlay (Cyan: normal parenchyma; Orange: lesion (fibrotic tissue); Green: basal collagen; White: white space; Blue: artefact). C and D: Magnified area within 1 lobe of PSR stained tissue with classifier overlay.
Once the tissue is segmented into ROIs, the third step involves quantifying PSR stain in both normal parenchyma and fibrotic tissue using the Halo Area Quantification App and then classifying the PSR stain into weak, moderate and strong intensity (see example in Figure 3). Strong PSR staining is associated with more mature collagen deposits including collagen fibrils, while weak PSR staining is associated with less well aggregated collagen and earlier collagen synthesis within the fibrotic lesion.
The separation of basal collagen components, such as bronchioles and prominent vascular structures, from the fibrotic lesion and normal parenchyma during the ROI classification stage, ensures that the collagen quantified within the sections is related directly to either the Fibrosis or Normal Parenchyma ROI, providing a precise evaluation of collagen changes across study groups within these areas of interest.
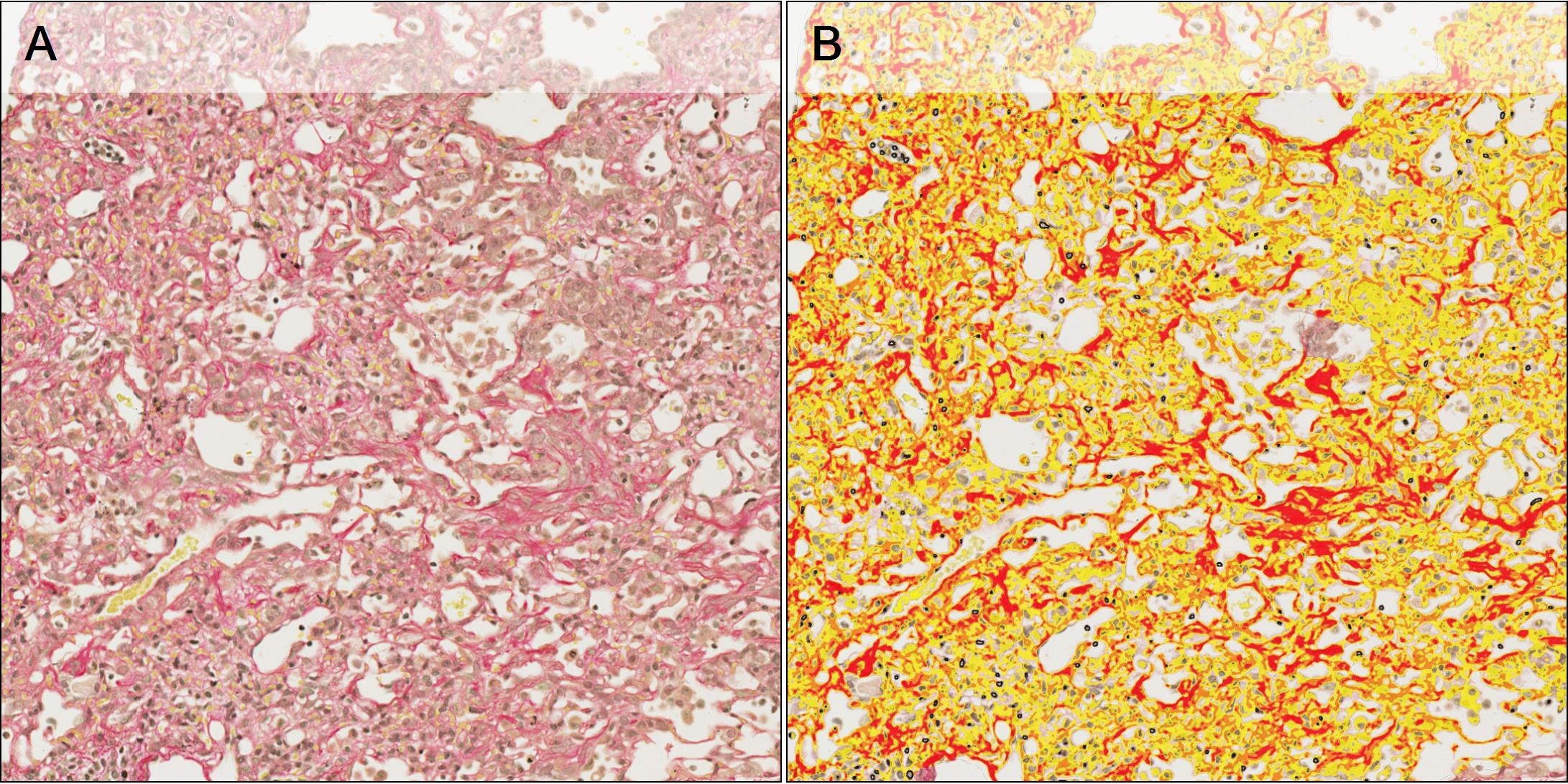
Figure 3 – A: Tissue section stained with PSR. B: PSR quantification overlay and classification into weak (yellow), moderate (orange) and strong (red).
To quantify the cell number per ROI, an additional algorithm is developed, using Halo AI Nuclei Segmentation classifier, to detect the individual nuclei present in each tissue (see examples in Figure 4).
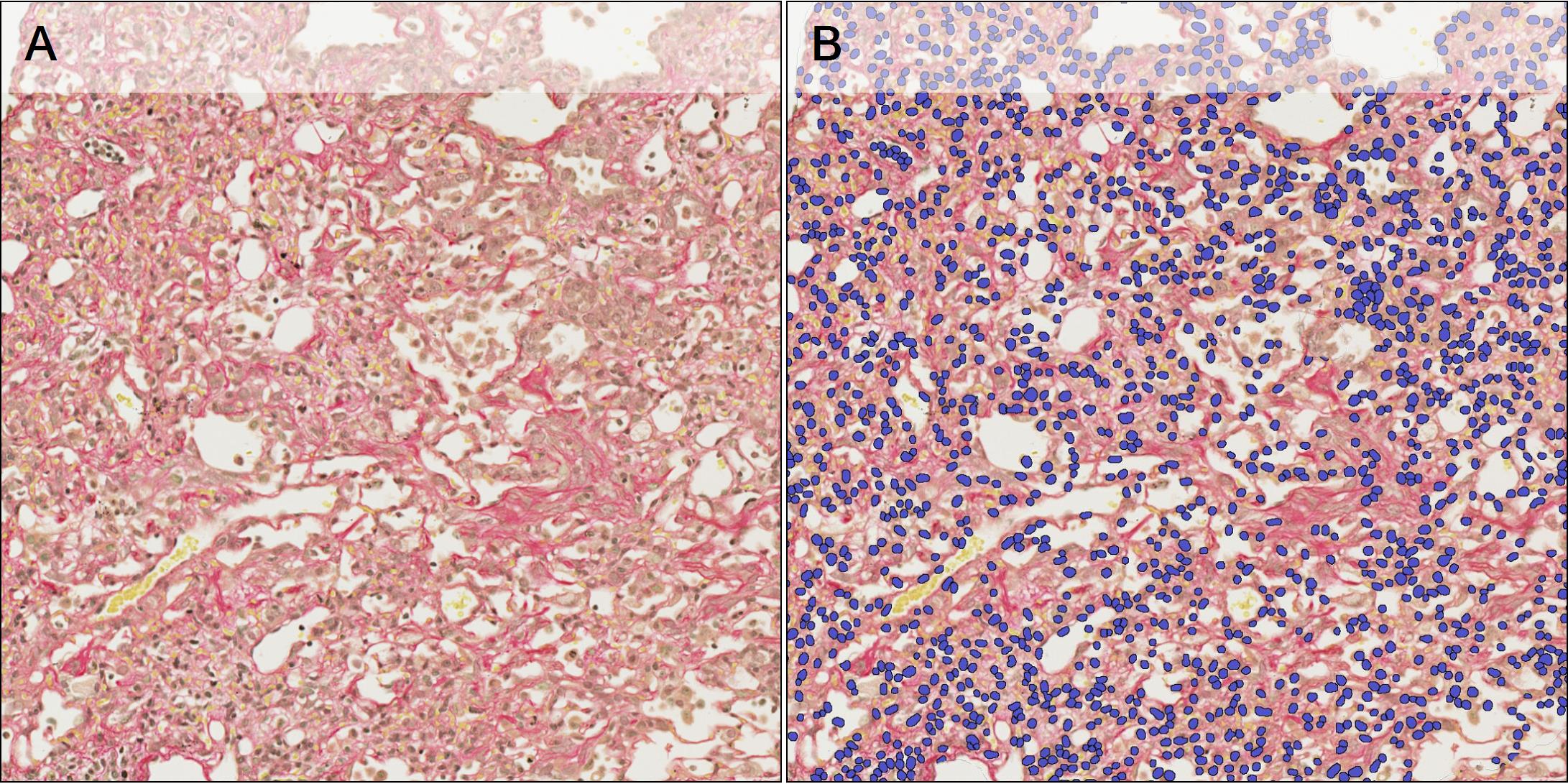
Figure 4 – A: Tissue section stained with PSR. B: Nuclei detection overlay (blue).
Example data that can be generated includes:
- Normal Parenchyma and Fibrosis ROI area per Section (in mm2)
- Total PSR positive area as a percentage of each ROI area
- Percentage of weak/moderate/strong PSR positive area in Normal Parenchyma and Fibrosis ROIs and relative H-score
- Cell density in Normal Parenchyma and Fibrosis ROI
In summary
For many drug development projects in IPF, the bleomycin model is a crucial step in the progression of the project to the next decision point. However, given the complexity of the model and its readouts, companies carefully need to consider, strategically, the project stage at which this model is performed and, operationally, how to best set this up including specifics of the model, dose levels, comparators, and outcome parameters.
While traditional biochemical assays have a considerable precedence, digital pathology has proven to be a great asset in studying lung fibrosis, from characterization to more detailed evaluation of response to treatment, enabling accurate interpretation of study outcomes complementary to the traditional approach. Also, adding image analysis could lead to a more holistic understanding of collagen biomarkers which could, eventually, offer a translational trajectory into early clinical development.
With the advances of AI, it is possible to estimate the degree of fibrosis with a precise and efficient quantitative method. Given their extensive potential in lung radiology and pathology, AI tools should continue to assist more in the future in the interpretation of lung images from histological specimens.
Working together to support you
With TherapeutAix and OracleBio’s combined expertise, we are ideally suited to support planning the bleomycin experiment itself as well as its analyses. At TherapeutAix, we have extensive experience with many providers of the bleomycin model and its different version, allowing us to not only recommend an optimal design but also the best provider. And with industry-leading image analysis expertise and processes, OracleBio is providing highest quality evaluations.
Despite its limitations, the bleomycin model continues to be an important data point and one that is required for most financing, licensing, or prioritization discussion.
If you’d like to get the best results from your bleomycin study, please get in touch to find out more or to schedule an initial discussion at the links below:
TherapeutAix:
- Website: therapeutaix.com
- Contact/Schedule a call: therapeutaix.com/contact-us
- LinkedIn: linkedin.com/company/therapeutaix
- Twitter: twitter.com/TherapeutAix
OracleBio:
- Website: oraclebio.com
- Contact/Schedule a call: oraclebio.com/contact
- LinkedIn: linkedin.com/company/oraclebio-limited
- Twitter: twitter.com/OracleBio
————
More about the companies:
TherapeutAix is a drug discovery consultancy company based in Aachen, Germany, combining research and development expertise with a fully integrated R&D network, to deliver practical, cost- and time-effective solutions to drug discovery projects. It supports investors and project teams focus on the next step, and progress the right assets through robust decision-making steps, with a clear line of sight to the next value-inflection point and the clinic. Since its formation in 2018, TherapeutAix has successfully conducted project reviews, developed strategies and operationalised programmes for biotech, pharma and investors in diverse therapeutic areas including fibrosis, inflammation, and respiratory disease.
OracleBio is a global leader in quantitative digital pathology based in Glasgow, Scotland, providing image analysis services to Pharma and Biotech clients worldwide. Leveraging multiple software platforms, the company delivers robust data packages within a quality management framework to support clinical trials and translational research. As image analysis experts, OracleBio specialise in cellular phenotyping of multiplex stained tissue and have built a strong reputation as the go-to company for complex image analysis.

