On International NASH Day 2019 we discuss the mixed outcomes of NASH drug clinical trials and how we believe a platform of evidence can be built to support future NASH drug development.
NASH (Nonalcoholic steatohepatitis) is emerging as a major public health issue for the 21st century that affects 7-12% of adults worldwide. It is associated with significant liver-related morbidity and morality and at present, there are no approved drug therapies for the disease.
Clinical trial readouts from the front-running NASH drug candidates so far are mixed, with obeticholic acid meeting primary trail endpoints, but selonsertib missing. These compounds, along with others in clinical development have had supportive Phase II data. However, further review of the data suggests that none of these compounds have shown consistent efficacy in the majority of patients.
We have discussed some of the reasons for these mixed outcomes in our recent review on NASH, in Drug Discovery Today. There are a number of pathophysiologies resulting in a common phenotypical pathology, which usually includes fibrosis, and is called ‘NASH’. The driving pathways behind disease progression can be metabolic, inflammatory, can result from disbalances in lipid metabolism or bile acid signalling, or from other liver injury.
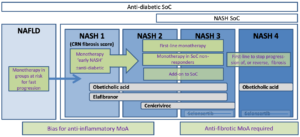
At TherapeutAix, we believe that the drug mode of action must match key pathophysiologic drivers, requiring a mechanistic understanding of efficacy and identification of patient segments. This allows specific drugs to show efficacy. The basis of this is a translational package supporting the envisioned positioning.
In addition, new therapies will need to be compatible with future standard of care for NASH, and also with many other medications prescribed to treat common co-morbidities.

